Total Magnesium
Clinical Applications
- Supports Cardiovascular Health*
- Supports Healthy Muscle Function/Healthy Nerve Conduction*
- Supports Bone Health*
- Supports Energy Production*
- May Support Healthy Glucose Metabolism*
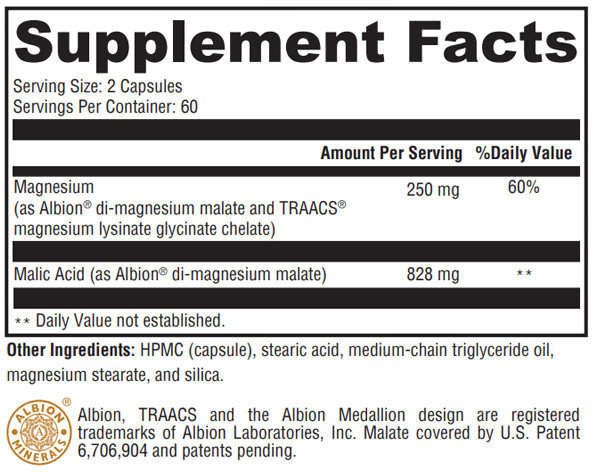
Magnesium, the fourth most abundant mineral in the human body, plays a role in over 300 metabolic processes. It participates in the development and maintenance of bones and teeth; the metabolism of carbohydrates, blood glucose, fats, and proteins; the formation of cells and tissues; and the maintenance of muscle function, including cardiac muscle. Total Magnesium contains Albion®’s TRAACS® magnesium lysinate glycinate (mineral amino acid chelate) and Albion’s chelated dimagnesium malate—both formulated for enhanced absorption. Malic acid (from di-magnesium malate) supports energy production and lactic acid clearance via the Krebs cycle. Malic acid may also support antioxidant systems by enhancing glutathione and antioxidant enzymes.*
All TheraSci, LLC Formulas Meet or Exceed cGMP Quality Standards
Discussion
Magnesium Lysinate Glycinate Chelate, a mineral amino acid chelate in which magnesium is bound to two amino acids, creates a complex that is more readily absorbed across the intestinal wall. Since the body can efficiently absorb dipeptides (two amino acids linked together), Albion’s TRAACS® magnesium lysinate glycinate is an excellent delivery system for magnesium. In general, Albion TRAACS patented mineral amino acid chelates are resistant to competitive minerals, do not weaken the action of vitamins, and pose a smaller risk of overdosing.*[1]
Di-Magnesium Malate, the other chelate in Total Magnesium, contains 69% malate (malic acid). Each capsule of Total Magnesium supplies approximately 400 mg of malic acid. Malic acid was chosen because it forms complexes with magnesium.[2] Magnesium and malate play critical roles in energy production under aerobic conditions or when oxygen is lacking.[3] Malic acid also appears to exert a protective effect by binding aluminum.*[4]
Magnesium, the fourth most abundant mineral in the body, participates in about 300-350 enzymatic reactions in nearly all tissues. Deficiency is common and results from poor dietary intake, poor absorption, and excessive losses through urine, stool, perspiration, or lactation. Certain drugs, certain herbs, poor kidney function, excessive alcohol intake, and drinking mostly “soft” water can contribute to magnesium depletion as well.*[5]
Magnesium’s role in the clinical applications cited above is quite well established. Beyond these commonly recognized applications, researchers have demonstrated that magnesium can support cytokine balance and decrease sensitivity to oxidative stress.[6] An analysis of the results of a National Health and Nutrition Examination Survey (NHANES) suggested that children who consumed less than 75% of the recommended dietary allowance (RDA) for magnesium were 58% more likely to have elevated C-reactive protein (CRP) levels.[7] Magnesium’s role in modulating CRP and supporting the body’s normal response to inflammation may be significant. In addition, although underlying mechanisms remain unclear, it appears that men who consume diets rich in magnesium are able to maintain healthy gallbladder function.[8] Adequate magnesium intake indeed
Directions
Consult your healthcare practitioner prior to use. Individuals taking medication should discuss potential interactions with their healthcare practitioner. Do not use if tamper seal is damaged.
Does Not Contain
References
1. Albion Minerals. http://www.albionminerals.com/human-nutrition/. Accessed May 23, 2012.
2. Schell J. Interdependence of pH, malate concentration, and calcium and magnesium concentrations in the xylem sap of beech roots. Tree Physiol. 1997 Jul;17(7):479-83. [PMID: 14759841]
3. Krinsky DL, LaValle JB, Hawkins EB, et al. Natural Therapeutics Pocket Guide. 2nd ed. Hudson, OH: Lexi-Comp; 2003.
4. Suzuki T, Tamura S, Nakanishi H, et al. Reduction of aluminum toxicity by 2-isopropylmalic acid in the budding yeast Saccharomyces cerevisiae. Biol Trace Elem Res. 2007 Winter;120(1-3):257-63. [PMID: 17916978]
5. Magnesium Balance: Can You Juggle? Albion Human Nutrition Research Notes. 2006 Dec;15(4). http://www.albionhumannutrition.com/research-notes/download/doc_details/328-magnesium-balance-can-you-juggle. Accessed May 29, 2012.
6. Scanlan BJ, Tuft B, Elfrey JE, et al. Intestinal inflammation caused by magnesium deficiency alters basal and oxidative stress-induced intestinal function. Mol Cell Biochem. 2007 Dec; 306(1-2):59-69. [PMID: 17657590]
7. King DE, Mainous AG 3rd, Geesey ME, et al. Magnesium intake and serum C-reactive protein levels in children. Magnes Res. 2007 Mar;20(1):32-6. [PMID: 17536486]
8. Tsai CJ, Leitzmann MF, Willett WC, et al. Long-term effect of magnesium consumption on the risk of symptomatic gallstone disease among men. Am J Gastroenterol. 2008 Feb;103(2):375-82. [PMID: 18076730]
9. Laires MJ, Monteiro CP, Bicho M. Role of cellular magnesium in health and human disease. Front Biosci. 2004 Jan 1;9:262-76. [PMID: 14766364
*These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.
